WHAT IS THE VALIDTIONMASTER PORTAL?
SHAREPOINT-BASED ENTERPRISE QUALITY, RISK AND COMPLIANCE MANAGEMENT SOLUTION FOR LIFE SCIENCES
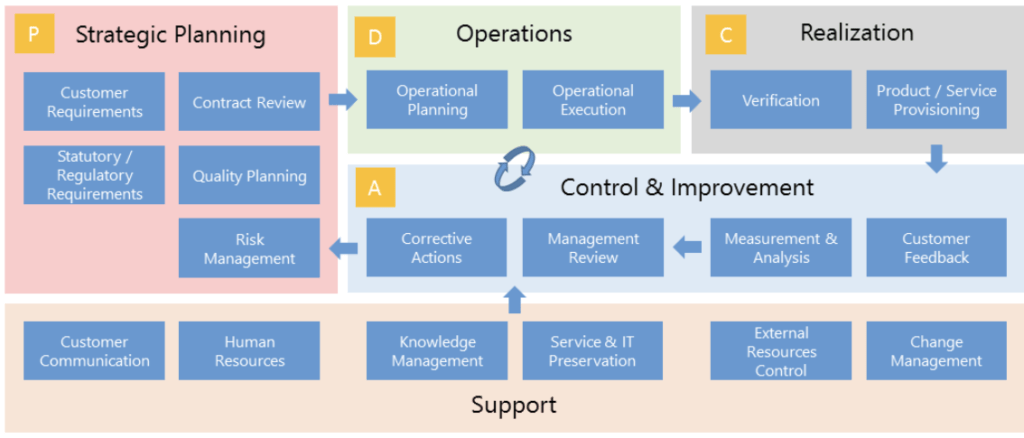
Validation Quality, Risk and Compliance
360 DEGREE INTEGRATED SOLUTION FOR TODAY’S QUALITY AND COMPLIANCE INITIATIVES

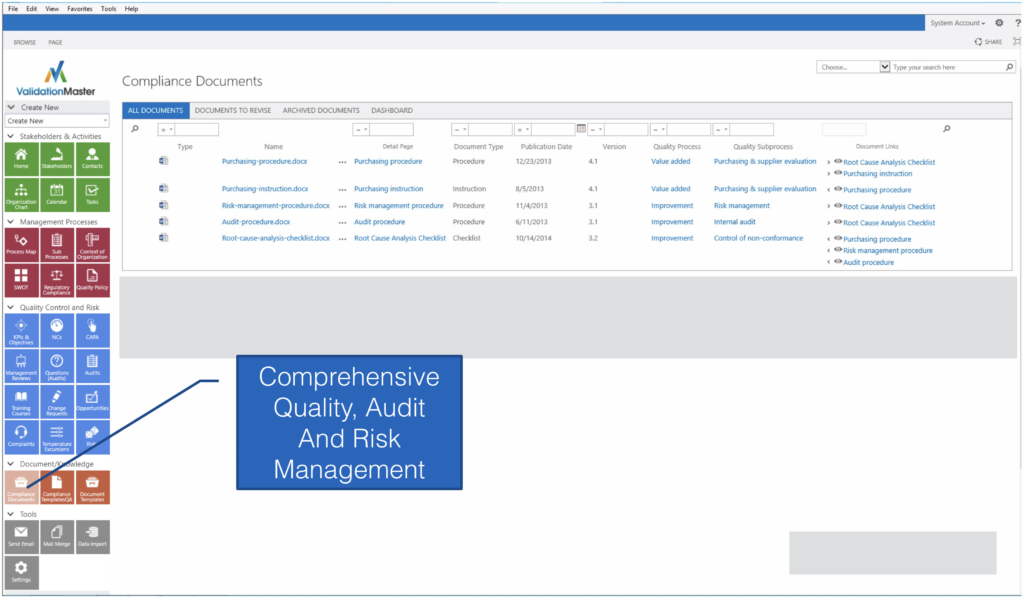
EASY TO CONFIGURE DOCUMENT REPOSITORY
BASED ON MICROSOFT SHAREPOINT®
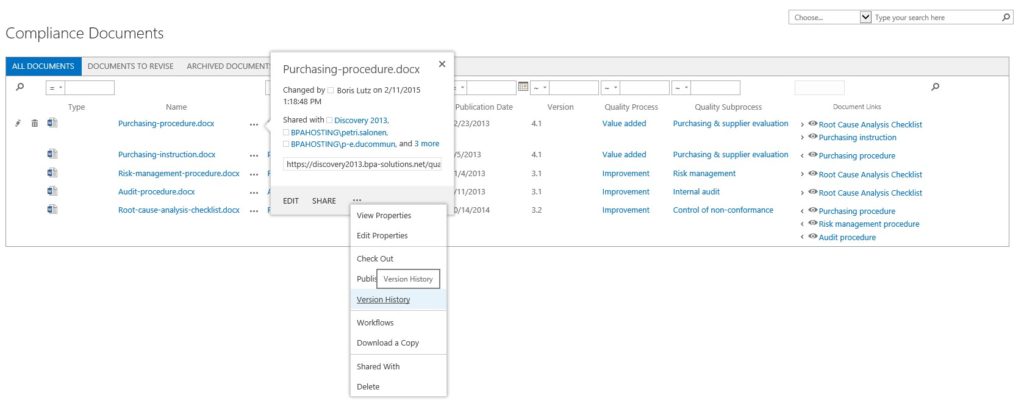
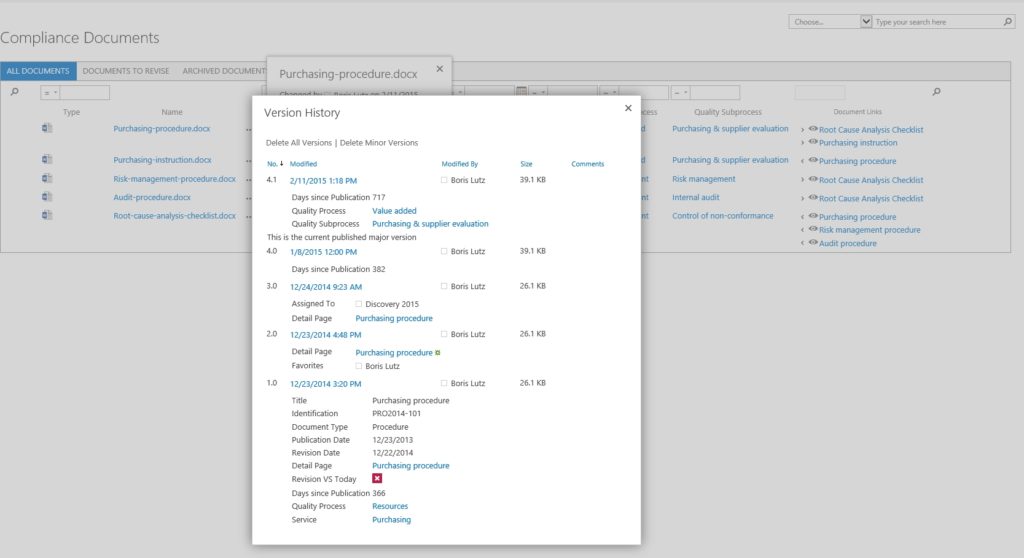
Comprehensive Document Version Control
Supports Major/Minor Release Numbering
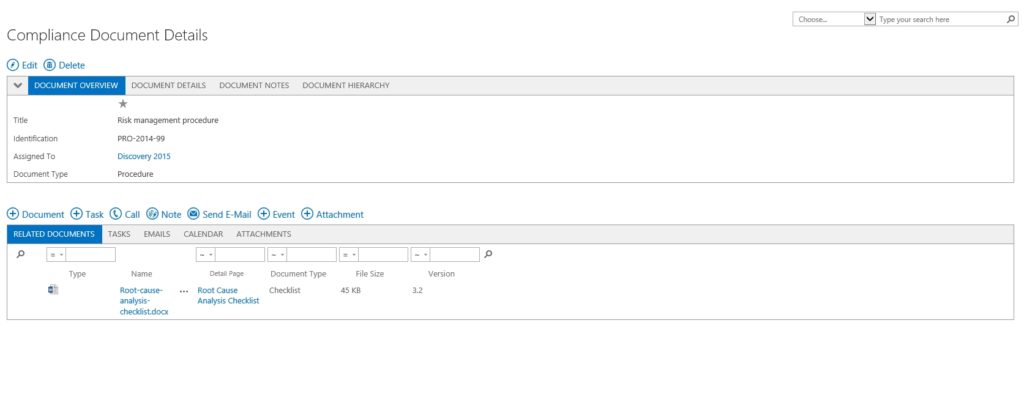
CONFIGURABLE DOCUMENT DETAILS
MANAGES BOTH CONTROLLED AND UNCONTROLLED DOCUMENTATION
Configure document details and the ability to manage tasks, events, attachments, email, notes and other attributes.
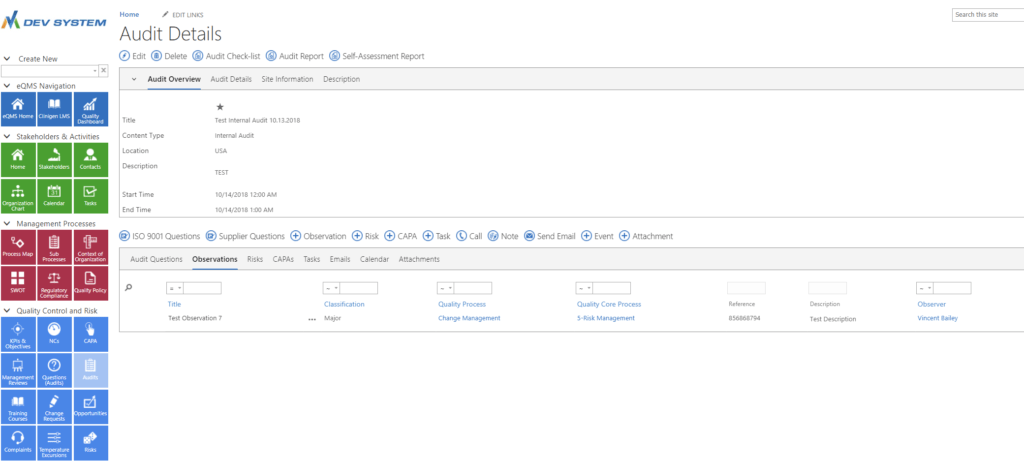
COMPREHENSIVE AUDIT MANAGEMENT
Create audits quickly ...
and assign questions, observations, risks, CAPAs, tasks, attachments and other information associated with the audit
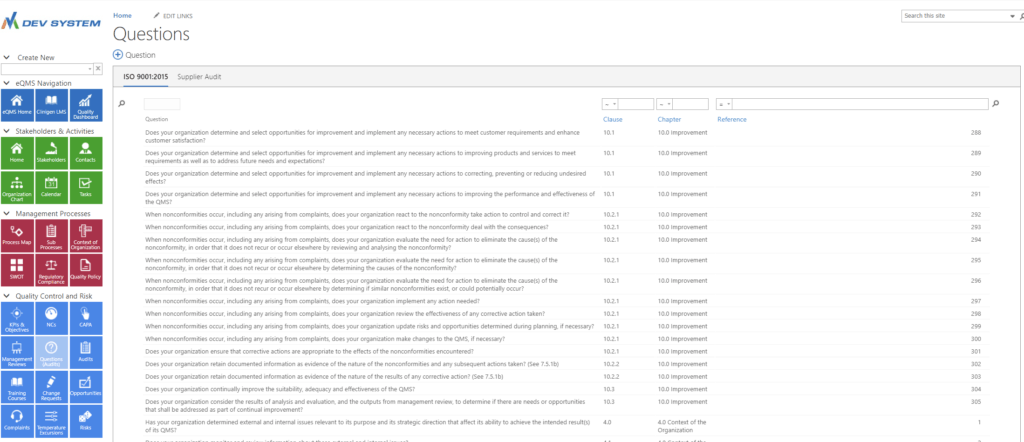
Create your own audit
Question library
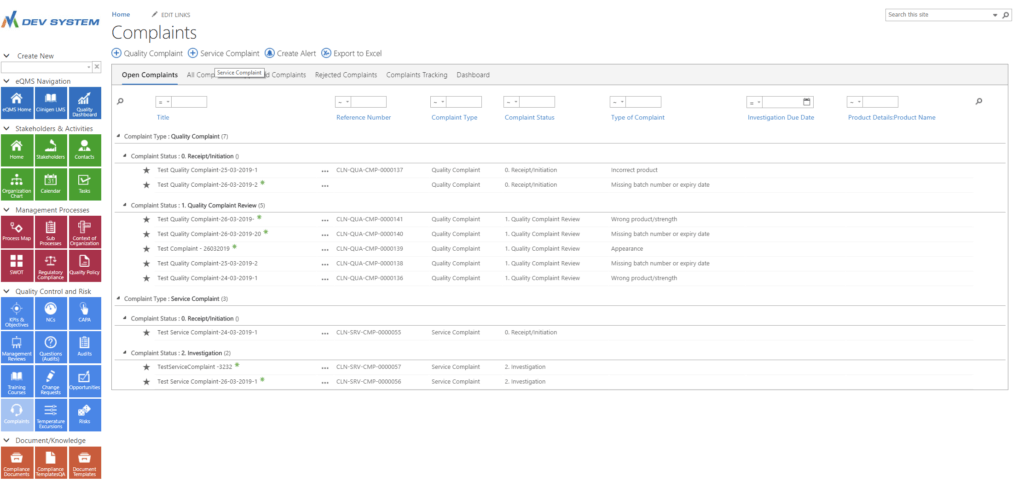
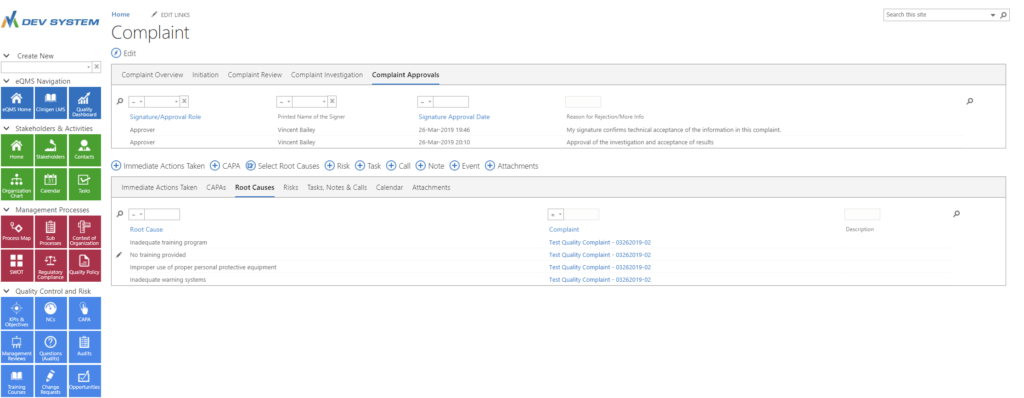
Manage quality
and service complaints
Electronically Approve Complaints
& Root Causes
Highlight root causes of complaints in the complaint details area.
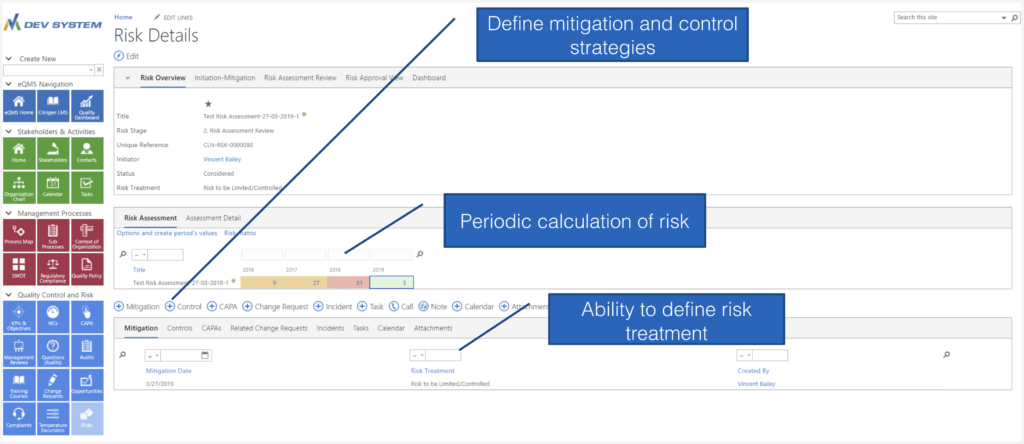
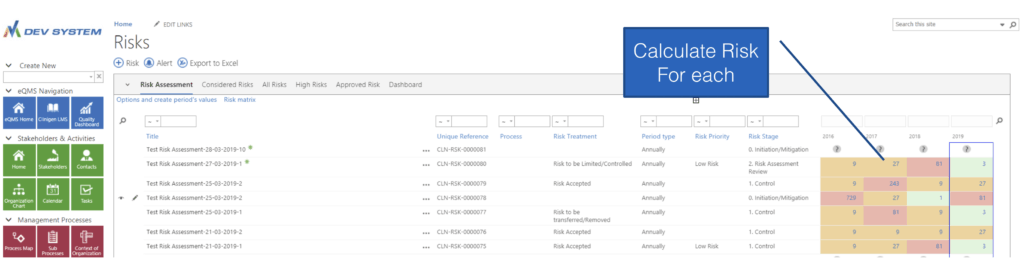
Comprehensive Risk Assessment
With Powerful Tools
Highlights Annual Risk Profile
Risk Calculation Dashboard
Graphical Workflow Processes!
Visually Create The Right Work Flows
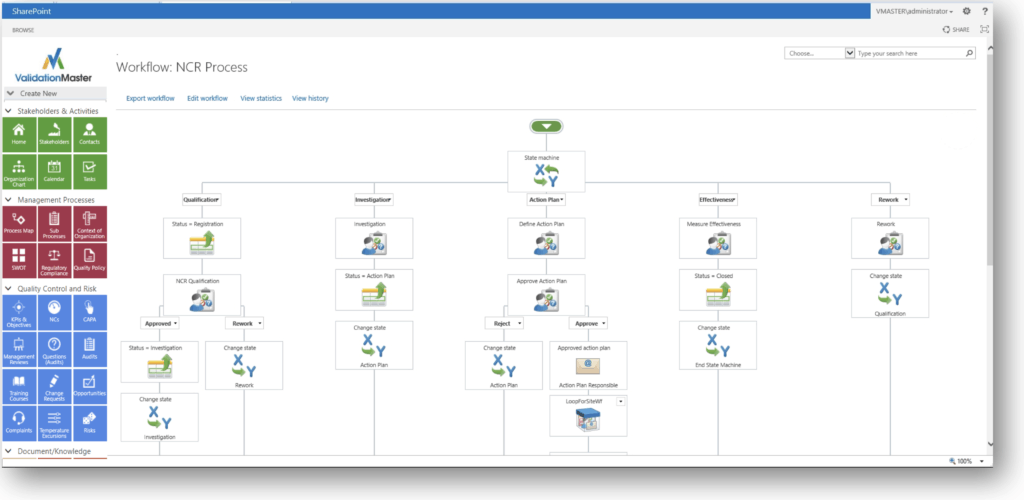
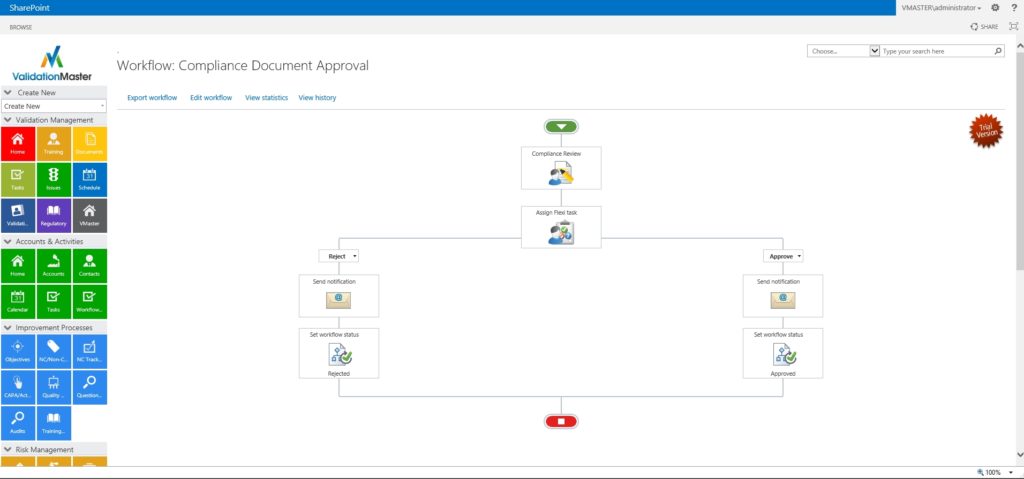
Compliance Document Approval
GRAPHICAL WORKFLOW
Powerful graphical compliance document workflow review and approval, Powered By Nintex.

Nonconformance Process
Graphical Workflows
User-definable quality workflows for NC, Compliance and CAPA processes.
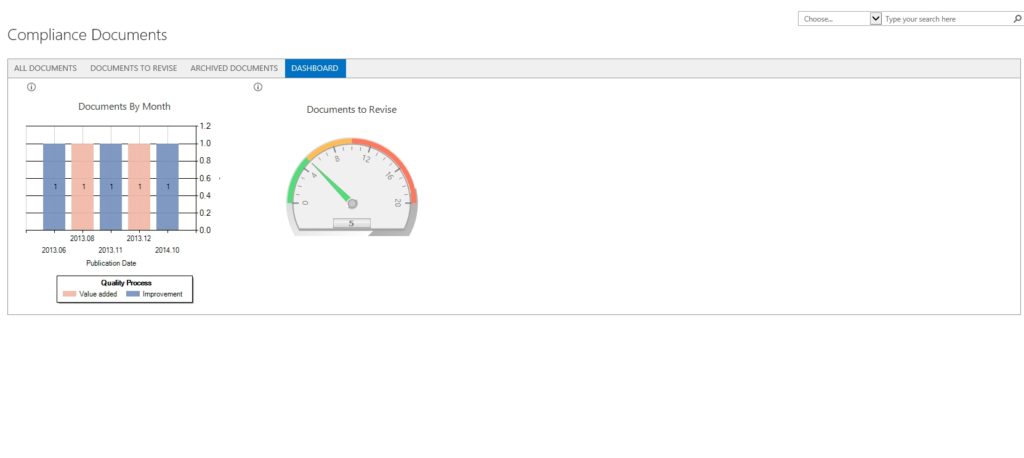
CONFIGURABLE DASHBOARDS
Unlimited Dashboards
You have as many configurable dashboards as you’d like. These configurable dashboards allow you to highlight real-time document processing metrics.
QUALITY &
Risk Management
VALIDATION CALENDAR,
KPIs & Dashboards
The system includes a rolling schedule for validation projects highlighting their status and periodic review dates. The system tracks validation KPIs and includes quality dashboards.
GRAPHICAL
Content Workflows
Powered by Nintex®, the portal includes graphical workflows to support document and change control, CAPA, N/C, and other workflow processes.
AUDIT
Management
The system includes a comprehensive audit management system in compliance with ISO 9001. You can setup pre-defined audit questions to build your own audit checklists and reports.

